Reimagining the Clinical Trial Experience
DURATION:
54 min
FORMAT:
Presentation
The costs of lost NIH-funded research alone due to COVID-19 is more than $10B. While there are no specific cost numbers from private clinical trials, nearly 1,200 clinical trials were either stopped or delayed due to COVID-19. Suspended enrollment was the root of many of these issues and with the cost of delay estimated between $600K-$8M a day in subsequent sales, the need to restart the trials and put patients back at their center is critical.
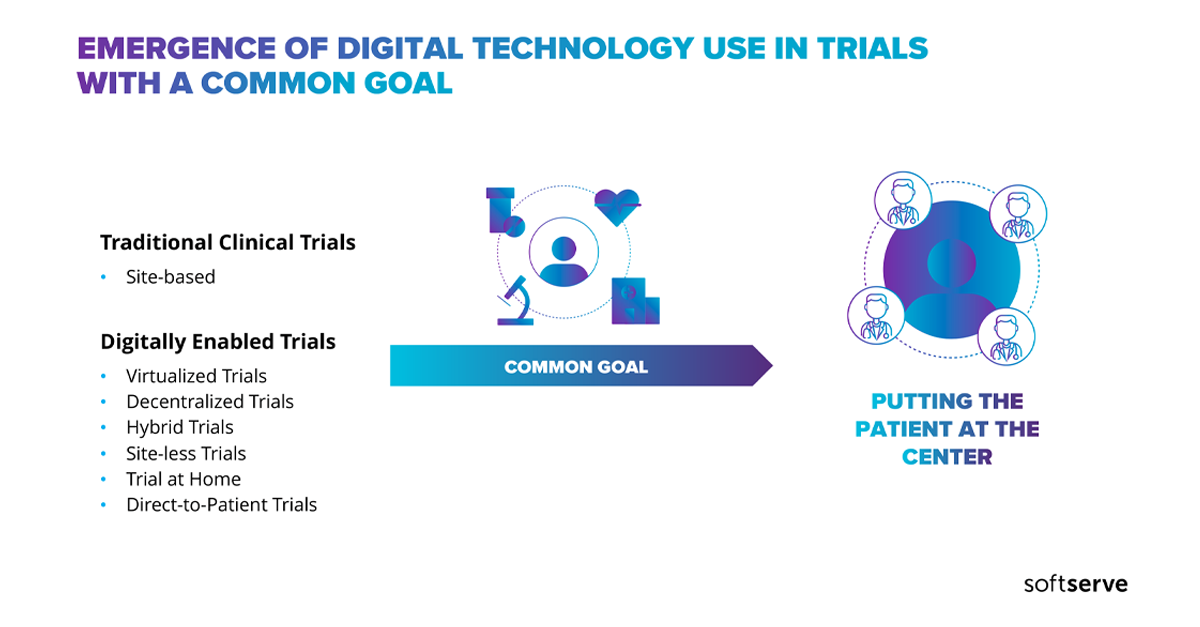
Key industry stakeholders have now seen the acute need to accelerate their adoption of virtual clinical trials. When it comes to clinical trials, adopting this model ensures future clinical trial continuity regardless of seen, or unseen, disruptions.
This digital event explains the key elements you need to set up and scale your digital health strategy successfully. You’ll learn how to expedite the move to virtual clinical trials with a modern digital infrastructure.
Gain insight into best practices for managing the expectations of sponsors, investigators, CROs, and patients. In addition, we detail how a purpose-built platform can create greater efficiencies and streamline key processes, which means you’ll achieve your clinical trial goals more quickly, with less risk and at a lower cost.
Watch and discover how to put patients back at the center of your clinical trials by integrating digital technologies into the clinical trial process.
Доступ до вебінару
Схожі записи
Engage with SoftServe’s industry and technology experts at meetups, conferences and online events

AI’s Role in the Future of Oil & Gas
Energy Sector,Energy, Oil & Gas,Tech Talks

Look Under the Hood: Edge Solutions in Practice
Manufacturing,Panel Discussion,Supply Chain

Navigating Edge Technology in Manufacturing
Manufacturing,Panel Discussion,Supply Chain

Beyond Automation: Unleashing the Power of IoT and Generative AI ...
Interview,Manufacturing



